Products Should Never Have Been Administered in Pregnant Women and Those of Childbearing Age
By Peter A. McCullough, MD, MPH
All drugs have a pregnancy category designation giving mothers and doctors guidance on what is known and how safe products are during pregnancy.
-
Category A: The possibility of fetal harm appears remote. Extremely few drugs exist in this category (e.g., multiple vitamins).
-
Category B: If there is a clinical need for a drug in this category, they are considered safe to use. Examples: acetaminophen, amoxicillin.
-
Category C: These drugs should be given only if the potential benefit justifies the potential risk to the fetus. Examples: fluoroquinolones, gentamicin, saccharin, aspirin.
-
Category D: There is positive evidence of human fetal risk, but the benefits from use in pregnant women may be acceptable despite the risk. They should only be used in pregnancy when the alternatives are worse. Examples: tetracyclines, ACE inhibitors, and most antineoplastics.
-
Category X: The risk of use of the drug in pregnant women clearly outweighs any possible benefit. The drug is contraindicated in women who are or may become pregnant. Examples: thalidomide, oral contraceptives, statins, all COVID-19 vaccines.
I published an article in 2021 with Dr. Raphael Stricker, who advises one of the largest fetal loss centers in the country, warning women that the COVID-19 vaccine should be considered pregnancy category X. This designation should have been assigned by the vaccine manufacturers and agreed to by the FDA and properly placed on all vaccine program documents since pregnancy and childbearing age without contraception were exclusions from the EUA registration trials.
Shockingly, in the very first week of mass vaccination in December of 2020, news reels depicted well-intentioned pregnant mothers getting injected with synthetic lipid nanoparticles laced with long-lasting mRNA coding for the Wuhan Institute of Virology Spike protein. How could this be happening with no mutagenicity or teratogenicity studies? How could good clinical practice by doctors be abandoned?
Pregnant mothers and vaccine center workers didn’t seem to care. There were no assurances of gestational, peripartum, or long-term safety. Would the baby be affected by this brand-new technology? The regulatory agencies and medical colleges appeared to be in a tranced oblivion.
There are more than a dozen papers that extoll the virtues of mass vaccination and quickly conclude that COVID-19 vaccines are “safe” in pregnancy. Each paper has critical flaws including: 1) not randomized, 2) no comparator group, 3) limited time window and not entire term with continued follow-up, 4) incomplete capture of clinical events, 5) conflict of interest with American College of Obstetrics and Gynecology (ACOG) who received and undisclosed sum of money from the White House HHS COVID-19 Community Core Fund to promote vaccination.
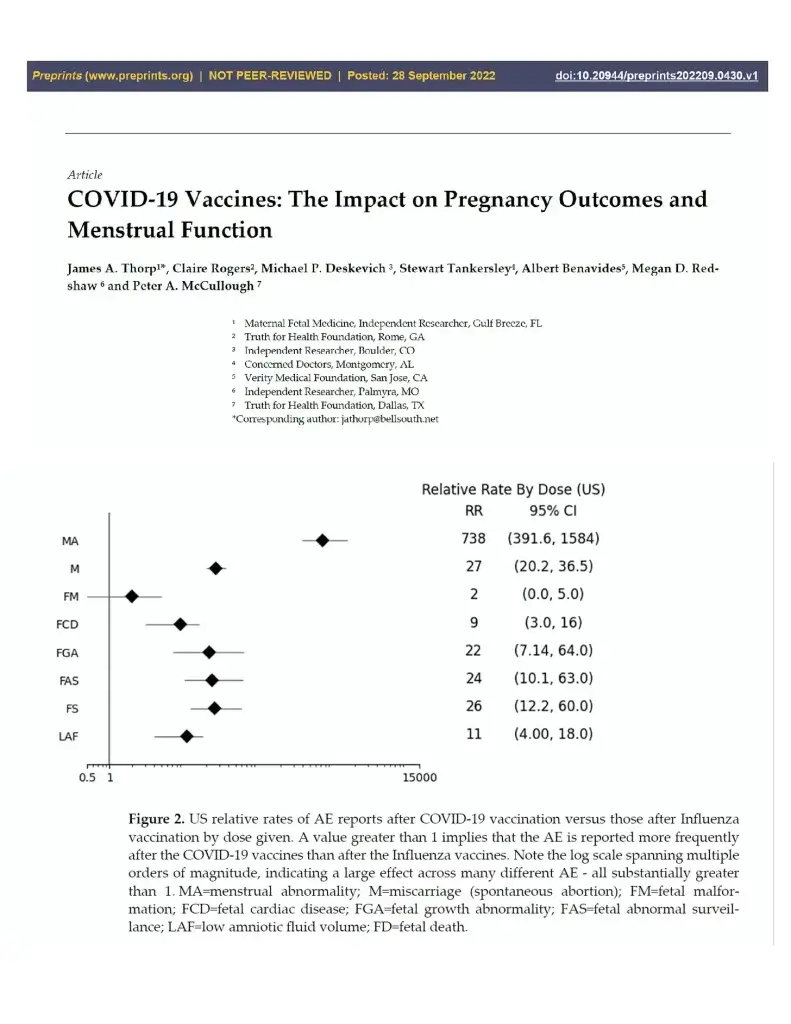
In drug safety research, even if it is only one abstract, manuscript, or monograph that raises a serious safety concern, it should be pursued over those which failed to find the signal. Thorp and colleagues used CDC VAERS and compared COVID-19 vaccines to influenza vaccines and found in US cases, a 27-fold higher risk of miscarriage and more than a twofold increased risk across six categories of adverse fetal outcomes. Because the mRNA is now known to circulate for a 28 days or more and the Spike protein causes clotting, bleeding, and is known to damage tissues, my conservative conclusion is that COVID-19 vaccination remains pregnancy category X—contraindicated.
If you find “Courageous Discourse” enjoyable and useful to your endeavors, please subscribe as a paying or founder member to support our efforts in helping you engage in these discussions with family, friends, and your extended circles.
- Why COVID-19 Vaccines are Pregnancy Category X by Dr. Peter McCullough | Nov 28, 2022 | Health, Politics
- Drugs in Pregnancy and Lactation. 8th Ed. Briggs GG, Freeman RK, Yaffe SJ Editors. Wolters Kluwer Health. Philadelphia. 2008.
- McCullough PA. Lack of Compelling Safety data for mRNA COVID Vaccines in Pregnant Women
- McCullough PA. mRNA Circulates at Least 28 Days after Injection Finding Cohesive with Serious Adverse Effects in First Month after COVID-19 Vaccination
- Thorp, J.A.; Rogers, C.; Deskevich, M.P.; Tankersley, S.; Benavides, A.; Redshaw, M.D.; McCullough, P.A. COVID-19 Vaccines: The Impact on Pregnancy Outcomes and Menstrual Function. Preprints 2022, 2022090430. https://doi.org/10.20944/preprints202209.0430.v1.